- English
- 简体中文
- Español
- Português
- русский
- Français
- 日本語
- Deutsch
- tiếng Việt
- Italiano
- Nederlands
- ภาษาไทย
- Polski
- 한국어
- Svenska
- magyar
- Malay
- বাংলা ভাষার
- Dansk
- Suomi
- हिन्दी
- Pilipino
- Türkçe
- Gaeilge
- العربية
- Indonesia
- Norsk
- تمل
- český
- ελληνικά
- український
- Javanese
- فارسی
- தமிழ்
- తెలుగు
- नेपाली
- Burmese
- български
- ລາວ
- Latine
- Қазақша
- Euskal
- Azərbaycan
- Slovenský jazyk
- Македонски
- Lietuvos
- Eesti Keel
- Română
- Slovenski
- मराठी
- Srpski језик
Pabrik China Kategori APIS.
- View as
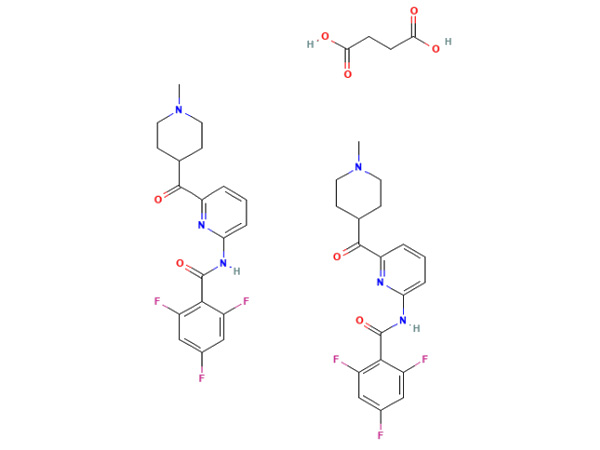
Lasmiditan Succinate
Lasmiditan Succinate nduweni spesifikasi In-house. DMF disetujoni.
CAS: 439239-92-6
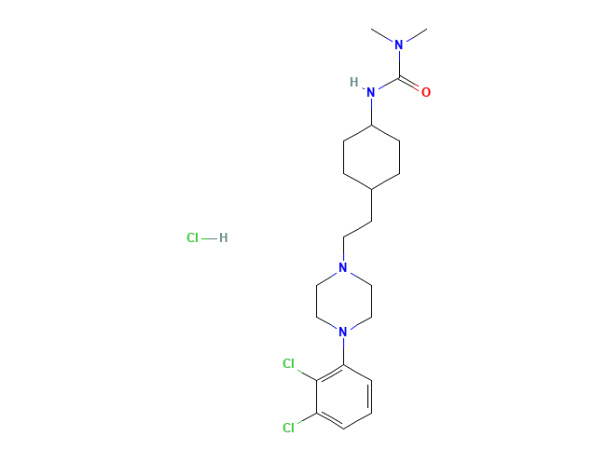
Waca liyaneKirim PitakonanCariprazin hidroklorida
Cariprazine hydrochloride nduweni spesifikasi In-house. DMF disetujoni.
CAS: 1083076-69-0
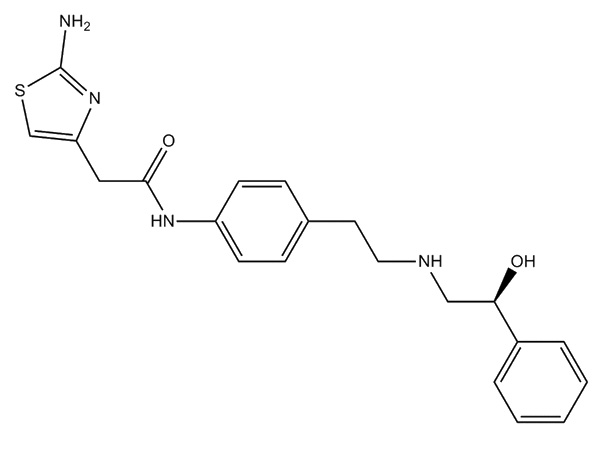
Waca liyaneKirim PitakonanMirabegron
Mirabegron nduweni spesifikasi In-house. DMF disetujui..
CAS: 223673-61-8
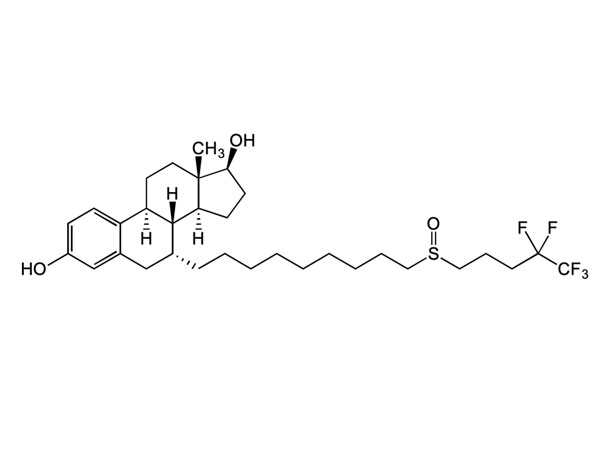
Waca liyaneKirim PitakonanLuliconazole
Luliconazole duwe spesifikasi ing omah, DMF disetujoni.
CAS: 187164-19-8
Waca liyaneKirim Pitakonan
Humanwell Pharmaceutical minangka salah sawijining pabrik API paling gedhe ing China. Kanthi luwih saka 20 taun pengalaman, kita berkembang, Pabrik lan perdagangan API steroid, intermediet lan formulasi. Pasar kita kalebu ing saindenging jagad, kita duwe kehadiran sing kuat ing Amerika Utara, Eropa, Amerika Selatan lan Afrika, kanthi produk sing didol menyang luwih saka 150 negara.